What Is Electroplating: Definition, Process, and Applications
- Plating |
- May 19, 2025

Electroplating is a widely utilized metal finishing and improvement technique used in metal stamping manufacturing industry to make the metal parts visually appealing and improve their efficiency.
Despite its prevalence, relatively few people outside the industry are acquainted with electroplating, what it is, and how it works. If you’re contemplating electroplating for your next manufacturing process, it’s critical to understand how the process works and the material and process alternatives accessible to you.
What is Electroplating?
Electroplating is a process that uses electricity to coat the surface of an object with a thin layer of metal. This technique is commonly used to improve appearance, prevent corrosion, reduce friction, and enhance other surface properties. The process involves depositing a thin metal layer onto a workpiece, known as the substrate, with the aid of an electric current. Electroplating can improve wear resistance, increase durability, and give objects a more polished look.
Electroplating was adopted and developed across Europe due to Brugnatelli’s studies. While electroplating may seem to be cutting-edge technology, the technique dates back to the Middle Ages. Electroplating was originally tested in the early 18th century, and Brugnatelli standardized the method in the first half of the nineteenth century. Over the following two centuries, as industrial processes progressed due to the Industrial Revolution and two world wars, the metal coating method changed to meet demand, culminating in the technology used by Sharretts Plating Company today.
Types Of Electroplating
Electroplating is essentially a metal coating technique that deposits a thin layer of material—such as gold plating or silver plating—onto another surface. This improves corrosion resistance, wear resistance, and electrical conductivity, making it valuable in protecting electrical components and decorative items alike. The types of metal coating can be classified by the process methods and by the metal method, are listed below.
Electroplating Types By Process Method
Rack Plating Method
The fixtures or a metal frame mount the individual metal parts on racks. The plating solution immerses the individual parts. Larger or complex parts also benefit from this approach, which ensures the best electric touchpoint for an improved electroplating process.
Pulse Plating Method
The pulse plating technique is an advanced electroplating method that applies current in pulses. The pulse pattern can be altered whenever needed as a waveform to manage the electroplating process steps. It functions both when the current is on and off.
Barrel Plating Method
Barrel plating is an electroplating method that coats a huge number of small metals all together. Plastic barrels rotate to coat the metal. This technique is best suited for screws, pins, or nuts. In addition, it is cost-efficient and easy to automate.
Electroplating By the Metal Plated Method
Nickel plating
Nickel plating adds a protective layer, improves the appearance, and increases the durability of household products, including doorknobs, flatware, and shower fixtures. The nickel electroplating method can be applied to both copper and aluminium. However, it performs admirably on other metals, giving chromium a basic covering.
Copper plating
This type of plating provides excellent conductivity at a relatively modest cost. Circuit boards and other electronic equipment are typically plated in copper. Since neither copper plating nor electroless nickel plating needs an external current, they both employ the same method.
Silver plating
Silver plating is an excellent metal finish choice if electrical conductivity is a crucial factor. For plating copper-based items, silver metal plating services are a cost-effective choice.
Gold plating
This technique is used in applications that call for great electrical conductivity and good oxidation resistance. Enhancing the conductivity of electrical connectors and other electronic components is just one of its many applications.
Electroplating Process Steps
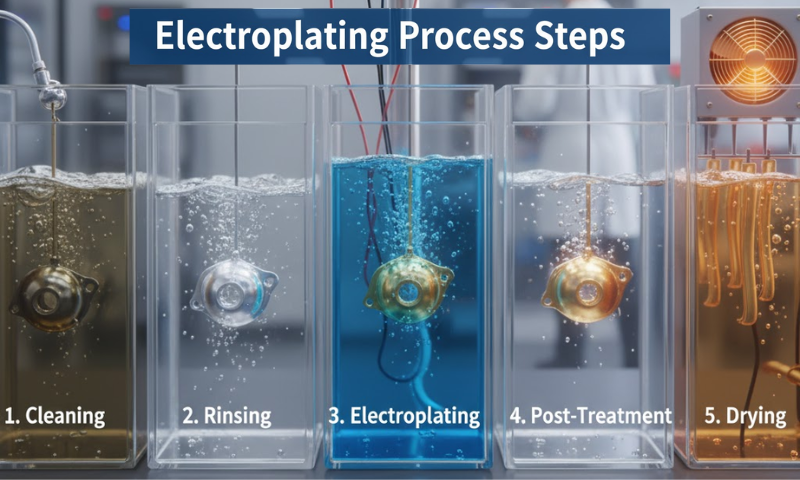
During the electroplating process, a metal dissolves in an electrolytic solution and deposits on a metal surface in the presence of an electric current. To explain the process of electroplating requires a few step-by-step processes. Below is a list of the method’s five crucial steps.
- Object Preparation: Initially, the object is cleaned to remove spots, dirt, grease, and waste particles. Basically, the chosen object must be metal.
- Electrolyte Process: The metal must consist of required ions. If the metal is copper, silver, or gold, it is necessary to specify the appropriate ion for each metal. For example, with silver or gold, it is necessary to specify the appropriate ion for that silver or gold. It is necessary to specify the appropriate ion that the particular silver metal requires for the electroplating process.
- Fix the Circuit: Connect the plated metal to the direct current in the negative terminal (cathode). The other metal part must be touched with the positive terminal (anode).
- Electrolysis: The current reduces the metal ions to increase the electrons. This method helps to make a sleek layer on the metal during the electroplating process.
- Final Polish: The electroplating process now produces thick metals. The object is removed from the process and washed and polished later.
Also Read- A Step By Step Guide For Copper Electroplating Solution
Materials Used in Electroplating
Coating Metal
The coating metal refers to the object, part of the vehicle, or metal that undergoes the coating process. The object metals are tin, gold, iron, nickel, and more. Each metal is used for specific purposes.
Electrolyte Solution
The metal plating needs metal ions that help the object reach an electrolyte solution. For example, to achieve copper plating, the copper metal requires copper sulphate.
Anode and Cathode
The positive shell is called an anode, and the negative shell is called a cathode. The electroplating cathode and anode play an essential role in the technique, where the copper metal needs copper electrons and the silver metal requires silver electrons.
Benefits Of Electroplating
Electroplating has several advantages for components. Among the many advantages of electroplating are the following:
- Protective barrier: Electroplating provides a barrier between the substrate and the environment. This barrier may, in certain situations, protect against corrosion induced by the environment. These distinct advantages because they endure longer in harsher environments and require less frequent replacement.
- Improved appearance: Exterior components are often coated with small layers of precious metals to increase their lustre and aesthetic appeal without high additional expenditures. It also protects silverware against tarnishing, increasing its lifespan and aesthetic look over time.
- Electrical conductivity: Silver and copper plating improve electrical conductivity in parts, providing a cost-effective and efficient method of conducting electricity in electronics and electrical components.
- Resilience to high temperatures: Several metals, notably gold and zinc-nickel, are resistant to high temperatures, enhancing the substrate’s resistance to heat damage. It, in turn, may extend the life of plated components.
- Increased hardness: Electroplating enhances the strength and durability of substrate materials. The coating makes them more resistant to damage caused by stress or harsh handling and assists in extending the life of the plated component.
Some advantages are metal-specific. Nickel plating, for example, is beneficial for minimizing friction, which helps to reduce wear and tear and extends the life of parts.
Other Metals and Chemicals
The extra chemicals used in the electroplating process include buffers, agents, levelers, and more. The additional chemicals help in providing the glossy appeal of the metal and a smooth finish.
Core Applications of Electroplating
Have you ever thought about what are the uses of electroplating? Here is your complete solution of electroplating applications and uses.
Ornaments
Electroplating is helpful when coating ordinary jewelry with real gold or silver. This procedure enhances the appeal and value. This method even makes a few imitation jewellery copper metal requires copper sulphate in order to achieve
Automotive Industries
The electroplating process enhances the visual appeal of automotive tools. For example, grilles, bumpers, bonnets, and other parts are designed to be resistance-free.
Medals, Shields and Coins
Electroplating is used to coat medals, coins, and shields so they look real and powerful. Essentially, designers create these for various tournaments and competitions.
Tool Manufacturing Industry
For longevity of metals, many industrial tools are electroplated with chromium after the tool manufacturing process itself.
Medical Tools
Some of the surgical tools and equipment are made to undergo electroplating to enhance biocompatibility and durability.
Households Tools
Household products such as restroom pipes, metals, cutlery, or kitchenware undergo electroplating to enhance their longevity and real appeal. Chromium and nickel are used to electroplate these tools.
Final Thoughts
To learn what is electroplating process is, it is necessary to understand its methods, uses, and types to explore each step of the plating process. Electroplating involves cathode and anode electrolyte solutions to coat the metal object. Are you looking for an eco-friendly electroplating service? Then, Eigen Engineering is the right place for you. They serve electroplating services worldwide with cutting-edge plating methodologies and eco-smart methods. Choose Eigen Engineering for reliability, expertise, and availability. Begin your journey with Eigen engineering for a productive electroplating service!
Ujjwal handles crucial roles like AGM Marketing, researcher, and is an author for KDDL – Eigen. He currently works with Eigen for implementing proven techniques and strategies for marketing plans on online and offline platforms. An expert in efficiently executing SEO, SEM, email marketing, social media marketing, PR marketing, Print campaigns, etc. Ujjwal has coordinated an efficient marketing team on various creative campaigns and programmatic buying to support various digital cross-promotion efforts. Implement efficient search optimization strategies with the help of collateral material and metrics.
In his former years, Ujjwal has years of experience in a managerial role for several reputed companies. His years of experience combined with the flair of writing help him come up with result oriented strategies for Eigen.